"Exploring Essential Types of Medical Equipment: A Comprehensive Guide"
From simple stethoscopes to advanced imaging systems, medical equipment underpins diagnosis, treatment, and monitoring across hospitals, clinics, and home care. This guide outlines key categories, common uses, and practical considerations relevant to care settings in Germany, with a focus on safety, training, and regulatory context.
Medical equipment spans a wide spectrum, from basic hand tools to complex life-support systems, each designed for a specific clinical purpose. In Germany, these devices are integral to daily workflows across hospitals, outpatient practices, rehabilitation centers, and home care. Understanding categories, safe operation, and regulatory expectations helps clinicians, caregivers, and procurement teams choose and use equipment responsibly. Patient safety hinges on correct setup, routine maintenance, and documented procedures that align with European and German standards.
This article is for informational purposes only and should not be considered medical advice. Please consult a qualified healthcare professional for personalized guidance and treatment.
Understanding how medical equipment is used
Medical equipment is defined by its intended use: diagnosing, preventing, monitoring, treating, or alleviating disease. In practice, this means devices must be operated within manufacturer instructions, with appropriate staff training and documented competencies. Before use, teams verify device readiness—checking calibration tags, battery levels, sterile status, and accessories. During use, they follow checklists and alarms, while after use they clean, disinfect, or sterilize according to risk level (non-critical, semi-critical, or critical items). Routine preventive maintenance and functional checks reduce downtime and help catch early signs of wear.
In Germany and across the EU, equipment must comply with the Medical Device Regulation (MDR) and bear CE marking to indicate conformity with safety and performance requirements. Many facilities also reference DIN/EN/ISO standards for testing, electrical safety, and reprocessing. Digital devices integrate with hospital information systems, so cybersecurity, data privacy, and audit trails are part of everyday use. Examples illustrate the range: a nurse programs an infusion pump with dose limits; a radiographer positions a patient for an X-ray using standardized exposure protocols; and a respiratory therapist adjusts ventilator settings guided by blood gas results and bedside monitoring.
A look at common medical equipment types
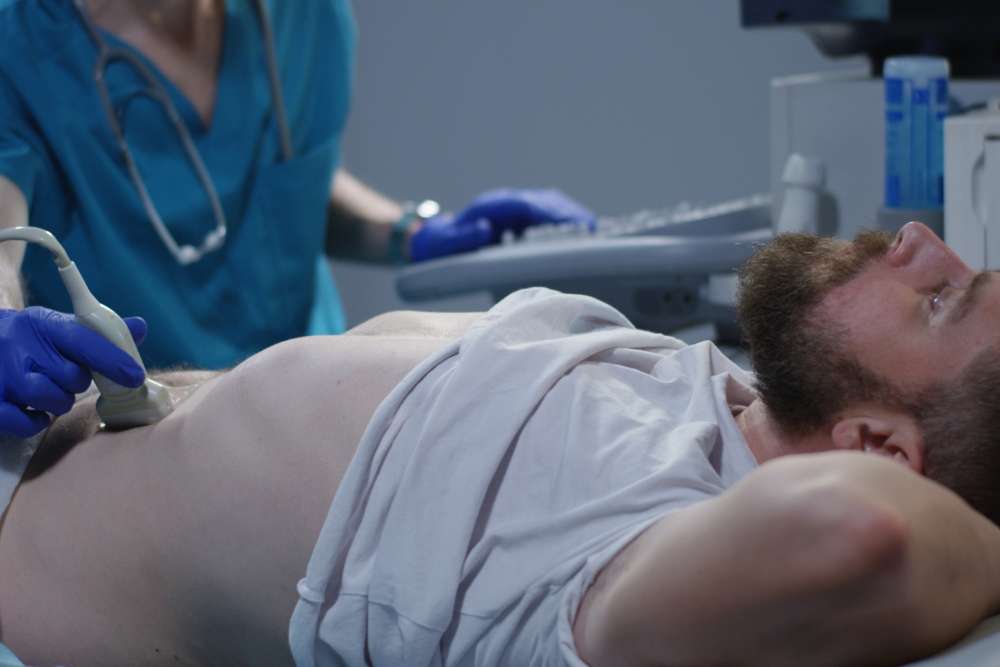
Diagnostic tools help identify conditions and guide decisions. Basic examples include thermometers, stethoscopes, and blood pressure monitors. More advanced systems encompass electrocardiographs (ECG), ultrasound scanners, X‑ray units, and laboratory analyzers. Point-of-care diagnostics—like glucometers and rapid tests—support decisions in emergency departments and community clinics. Imaging and lab devices typically require regular calibration and quality controls to maintain accuracy.
Therapeutic equipment delivers treatment. Infusion pumps administer medications and fluids with precise flow control; defibrillators provide electrical therapy for cardiac arrest; ventilators support breathing in intensive care and transport settings; dialysis machines substitute kidney function; and surgical devices such as electrosurgery units and lasers enable precise tissue management. Each device type carries specific safety considerations, such as infusion drug libraries to prevent dosing errors or grounding checks for electrosurgical systems.
Monitoring and support equipment sustain patient stability and comfort. Multiparameter monitors track heart rate, blood pressure, oxygen saturation, and respiratory rate at the bedside. Capnography and invasive pressure monitoring appear in operating rooms and ICUs. Mobility and care-support items include wheelchairs, patient lifts, hospital beds with adjustable rails, and pressure-relief mattresses. Infection prevention relies on sterilizers (autoclaves), washer-disinfectors, and personal protective equipment (masks, gloves, gowns). Accessories—electrodes, tubing, filters, and single-use kits—must be compatible and documented as part of the device system.
General insights into medical device applications
Applications vary by setting. Hospitals rely on high-acuity systems—anesthesia workstations, imaging suites, ventilators—while ambulatory practices emphasize compact diagnostics and minor-procedure tools. Home care uses portable devices such as nebulizers, blood pressure monitors, pulse oximeters, and home dialysis solutions, with instructions tailored for non-professional users. Rehabilitation and long-term care focus on mobility aids, pressure management, and monitoring for chronic conditions.
Connectivity and data handling are increasingly important. Devices may exchange data using standards like DICOM for imaging or HL7/FHIR for clinical records, enabling clinicians to view results across systems. With connected devices, facilities consider role-based access, software updates, and secure wireless networks. In Germany, data processing must comply with GDPR, and facilities often implement strict governance for device inventories and logs.
Procurement and lifecycle management aim to balance clinical need, safety, and cost of ownership. Typical steps include needs assessment, vendor evaluation, technical and safety testing, user training, and acceptance checks. After installation, asset management tracks maintenance intervals, software versions, and incident reports. Clear labeling, unique device identification (UDI), and up-to-date manuals support traceability and recall handling. Environmental factors also matter: energy-efficient imaging, reusable accessories where appropriate, and compliant disposal of batteries, electronics, and sharps can reduce environmental impact.
Training and competency underpin safe application. Simulation-based exercises help teams practice rare events, like defibrillator use or airway emergencies. Standard operating procedures and quick-reference guides support consistency during shift changes. For home users and caregivers, education focuses on correct setup, recognizing alarms, cleaning, and when to seek professional help. Manufacturers’ instructions and facility protocols should always be the authoritative references for operation and reprocessing.
Selecting equipment benefits from structured evaluation: define clinical requirements, review evidence of performance, consider interoperability with existing systems, and assess service availability in your area. For devices that contact the body or deliver therapy, check for compatibility with current consumables and infection-control practices. For connected devices, verify data formats, cybersecurity posture, and update pathways. Ensure that service agreements cover preventive maintenance, calibration, and rapid troubleshooting to minimize downtime.
Conclusion Medical equipment forms the backbone of modern care, linking accurate diagnosis with effective treatment and reliable monitoring. Successful use depends on matching devices to clinical need, adhering to safety standards, training users, and maintaining equipment throughout its lifecycle. With careful selection and consistent processes, healthcare providers and home users can support safer, more efficient care across German settings.